RA Clinical Trials: What is the Purpose of Clinical Trials?
In order to develop the most effective, efficient and safest methods for diagnosing, preventing treating, and understanding diseases, physicians, scientists and researchers hold clinical trials. Patients actively participate in controlled health assessments and treatment testing in order for medications and devices to be assessed and eventually approved by the Food and Drug Administration (FDA).
Clinical trials are an integral part of disease and treatment research. Some of the most advanced findings in research and treatment have resulted from successful clinical trials. To continue to develop the most competent and safest long-term treatments for rheumatoid arthritis (RA), clinical trials are held all over the world for participating RA patient volunteers.
What are Clinical Trials?
Clinical trials are controlled assessment and research environments hosted in different locations around the world that focus on one particular idea regarding a disease or condition. Patient volunteers are assessed and selected to participate in a clinical trial.
The goals of the clinical trials can vary from disease research and diagnostic analyses to looking for preventative measures and finding the best possible treatment options.
Here are examples of types of activities that happen at clinical trials:
- New drug treatments
- Combination drug treatments
- Treatment using new surgical procedures
- Treatment using new medical devices
- New uses of existing forms of treatment
In addition to clinical trials, researchers also hold clinical research sessions, which may focus less on treatment and more on understanding the underlying components of the disease. These sessions tend to concentrate on how to diagnose and prevent the disease. Here are some of the activities that happen during clinical research:
- Scientific medical investigations on individuals or groups of patients
- Studies on personal medical backgrounds and family histories to find common diagnostic criteria or patterns
- Disease progression studies
- Disease complication studies
How do Clinical Trials Work?
Clinical trials start with an initial idea of what the researchers want to better understand and safely test. New treatments that have proven successful in a laboratory setting can be moved to clinical trials for active patient participation.
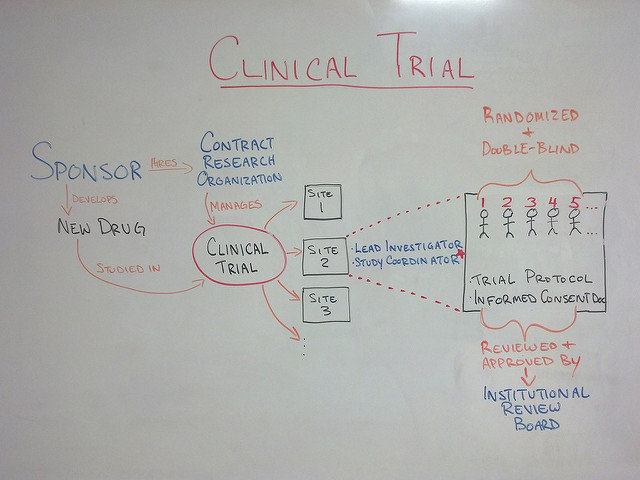
A Principal Investigator (PI) who is usually a physician leads the clinical trials. Research team members monitor results and assess safety and efficacy of the intervention being studied.
In addition to the research team, an Institutional Review Board (IRB) usually approves the clinical trial and oversees its progress to protect patients and reduce risks. In order to begin a clinical trial, a protocol (or structured plan) as to how the study will be conducted and the potential benefits that will be gleaned from the trial must be submitted to the IRB for review. The IRB may approve, make adjustments or reject the proposal. The following is the general protocol that clinical trials follow:
- Decide patient eligibility for participation
- Disclose testing procedures, medications to be used, and their dosages
- Provide information about the study length and the information that will be collected
- Disclose all potential risks to the participants
- Provide plans for monitoring for side effects or complications of the study
- Hypothesize potential benefits that may result from the study based on prior laboratory data
Once the design for research testing is drafted and approved by the IRB, the researchers must follow this protocol to select the appropriate candidates. This protocol states which patients are eligible to participate and is often based on age, sex, and the stage of the disease (among other characteristics of the patient and the disease).
The protocol also includes specific regimens for the types of treatment, medication information, and dosages that will be provided during the clinical trial. The plan also outlines how long the study will run for, and the information that will be gathered during the study.
Patient care is the driving force behind clinical trials. The purpose of disease research is to ensure that the highest levels of patient care are safely and effectively made available to all those in need.
Why Clinical Trials are Important
Clinical trials are a crucial catalyst between disease research and the availability of treatment options. The findings of clinical trials lead to scientific discoveries about how to not only effectively treat the disease, but often how to diagnose and prevent the disease altogether.
Patients who participate in clinical trials report that they feel their participation is contributing to the positive advancement of science and clinical practice. They not only want to help others, but they also understand the possibility that they will receive the most “cutting edge” treatments and medical attention currently available.
Clinical trials offer support and hope to patients and their families as well as others around the world who may need treatment in the future.
Rheumatoid Arthritis Clinical Trials
Because RA has no known exact cause and no known cure, clinical trials are extremely important in helping to better understand the disease. RA clinical trials discover new information about the underlying disease process, as well as how to better diagnose it and how it progresses.
Of course, one of the most important aspects of RA clinical trials is finding safe and effective treatments that ideally stop or slow the disease’s progress. New treatments are currently being tested that focus on advanced types of disease-modifying antirheumatic drugs (DMARDs) including immunotherapies and biological response modifiers.
RA clinical trials are available to select RA patients. To find out about the available clinical trials, you must first discuss the opportunities with your physician. Your physician can discuss with you disease registries that you qualify for as well as refer you to ongoing clinical trials that you can be a part of.
What to Know About Participating in Rheumatoid Arthritis Clinical Trials
Being part of a clinical trial can be a rewarding and fulfilling experience that helps with indispensable scientific discoveries. If you are considering participating in a clinical trial, there are some important things to know before you proceed.
Below are some things to consider and understand before participating in a RA (or any) clinical trial:
- Understand clearly the potential risks including the chance and degree of harm that can occur
- Know the required commitment including length of time, blood testing, hospital stays and more
- Thoroughly review the informed consent document prior to signing the agreement
- Fully discuss the risks and benefits with your physician and family
- Educate yourself on the benefits of the clinical trial including access to new treatments and therapies
- Discover if there are any costs associated with participating in the clinical trial
- Have a positive mindset about your participation and your ability to help others
Participating in clinical trials gives you the chance to receive additional medical attention and support. Be sure to ask plenty of questions along the way including:
- Why the study is important?
- What potential benefits could you experience?
- How is the study being funded?
- Who is overseeing or reviewing the study? (IRB or private firm?)
- What are the safety concerns?
Clinical trials are held to high safety standards and ethical guidelines. Though there are potential risks, there are also tremendous benefits. Be sure to weigh the risks and benefits to ensure you feel confident before moving forward with participating in a clinical trial.
